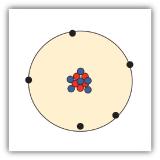
All matter is composed of atoms; atoms of the same element are the same, and atoms of different elements are different; atoms combine in whole-number ratios to form compounds.
A proton is larger than an electron.
proton: 1+; electron: 1−; neutron: 0
In the nucleus of the atom